Important Questions for Class 11 Biology Chapter 9 Biomolecules are available here for students to boost their exam prepration. These questions are prepared by subject experts and created exclusively for exam preparation. By practising these questions, students will cover all the important topics and revise the entire chapter once again. Also, they could able to handle any type of question asked from this chapter in the examination.
Important Questions for Class 11 Biology Chapter 9 Biomolecules
Though there is diversity in living entities, they share similarities in the chemical composition and metabolic reactions. When examined quantitatively, the elemental composition of non-living and living matter appear to be similar. On the contrary, close analysis unfolds that the comparative abundance of hydrogen, carbon, and oxygen is much more in living entities in comparison to the non-living matter, out of which most abundant chemical found is water in living entities. Some organic compounds found in living entities are monosaccharides, amino acids, fatty acids, glycerol, sugars, nucleosides, nucleotides, etc. Read on to know more about biomolecules.
Very Short Answer Type Questions
Q.1. Classify the following based on whether they were initially received as a natural product or as a synthetic chemical.
a) Penicillin b) Sulfonamide c) Vitamin C d) Growth hormone
A.1. Listed below is the classification
| Natural product | Synthetic Chemical |
| Penicillin | Sulfonamide |
| Vitamin C | – |
| Growth hormone | – |
Q.2. Classify the following into one of the appropriate bonds – ester bond, peptide bond, glycosidic bond, hydrogen bond.
a) Polysaccharide b) Protein c) Fat d) Water
A.2. a) Polysaccharide – glycosidic bond
b) Protein-peptide bond
c) Fat – ester bond
d) Water – hydrogen bond
Q.3. Name any one sugar, amino acid, fatty acid, nucleotide.
A.3. Sugar – Lactose
Amino acid – Leucine
Fatty acid – Palmitic acid
Nucleotide – Adenosine
Q.4. How are co-factors different from prosthetic groups?
A.4. Prosthetic groups are organic compounds whereas cofactors can be organic or inorganic (metal ions). Prosthetics are distinguished from cofactors as they are tightly bound to the apoenzyme.
Q.5. Chitin, Cellulose, Glycogen, Polysaccharides and Starch are present in the following options. Choose and write appropriately against each.
a) Cotton fibre b) Exoskeleton of Cockroach c) Liver d) Peeled Potato
A.5. a) Cotton fibre – Cellulose
b) The exoskeleton of Cockroach – Chitin
c) Liver – Glycogen
d) Peeled Potato – Starch
Q.6. Alanine and Glycine are different with regards to one substituent on the a-carbon. Mention other common substituent groups.
A.6. The common substituent groups are – H and NH2COOH.
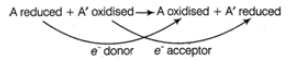
Q.7. Oxidoreductase catalyzes the following reaction between substrates A and A’, complete the reaction
A reduced + A’ oxidized →
A.7. The enzyme oxidoreductase catalyzes the transfer of e– from the reductant, known as the electron donor to the oxidant, known as the electron receptor. The reaction is as follows:
Q.8. What are Biomolecules?
A.8.Biomolecules are the essential organic molecules, primarily involved in the maintenance and metabolic processes of living organisms.
Q.9.Which is the most abundant element found in living organisms?
A.9.The most abundant element in living organisms is water.
Q.10.How many types of biomolecules are there?
A.10.There are four major classes of Biomolecules – Carbohydrates, Proteins, Nucleic acids and Lipids.
Short Answer Type Questions
Q.1. The functional groups in amino acids are weak bases and acids chemically, the ionization is affected by the pH of the solution. The activity for several enzymes is affected by the ambient pH and is depicted in the curve below, explain in brief.
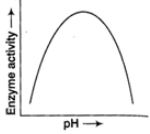
A.1. Enzymes usually operate in a narrow range of pH. Most of them indicate their highest activity at a pH known as the optimum pH and decline above and below this value. Extremely low or high pH normally leads to complete loss of activity for most of the enzymes. The graph indicates the maximum activity of the enzymes at the optimum pH.
Q.2. Can rubber be classified as a primary metabolite or a secondary metabolite? Write a short note on the rubber.
A.2. It is a secondary metabolite and is extracted from the rubber tree ( Hevea brasiliensis). Latex in the rubber tree is produced by the highly specialized cells in phloem called laticifers. Rubber is a terpenoid and because of its high tensile strength, plasticity, and elasticity, it is used across industries. It is a polymeric substance and is a good conductor of electricity.
Q.3. Justify with the help of an example of why nucleic acids display secondary structure.
A.3. The secondary structure of a nucleic acid molecule is in context of base-pairing interactions in a single molecule or a group of interacting molecules. The secondary structure of RNA and DNA vary. For example, the secondary structure of DNA consists of two complementary strands of polydeoxyribonucleotide, that are coiled spirally on a common axis forming a helix. This double-stranded helical structure of the DNA is supported by the phosphodiester bonds, ionic interactions, and hydrogen bonds.
Q.4. The living state is a non-equilibrium steady-state to be able to perform work – Comment.
A.4. Living entities exist in a steady state that is characterized by the concentration of every biomolecule, which is in metabolic flux. Any physical or chemical phenomena move parallelly to the equilibrium. Living entities work continuously hence they cannot reach equilibrium. Thus living state is in a non-equilibrium steady-state to be able to perform work that is attained via energy provided by metabolism.
Q.5. What are the sources of Proteins?
A.5. The most common food which has a high in protein are almond, beans, broccoli, chicken, cottage cheese, eggs, fish, milk, oats, pulses, seafood, soy, quinoa and yoghurt.
Long Answer Type Questions
Q.1. In catalyzed reactions, the formation of the enzyme-substrate complex is the first step. Explain the other steps until the formation of the product.
A.1. The cycle of enzyme action can be explained as follows:
- The substrate binds to the active site of the enzyme first, that fits into the active site
- This binding causes the enzyme to change its shape, leading it to fit more tightly around the substrate
- In the vicinity of the substrate, the active site of the enzyme breaks the chemical bonds of the substrate leading to the formation of the new enzyme-product complex.
- Products of the reaction are released by the enzyme. The free enzyme is now available to bind to another such molecule of the substrate and enter into the next catalytic cycle.
Q.2. Explain through the Watson-Crick model, the secondary structure exhibited by the nucleic acids.
A.2. Nucleic acids display a wide variety of secondary structures. For example, the Watson-Crick model. It proposes that the DNA exists as a double helix structure, wherein the two strands of the polynucleotides run in the opposite direction. Its backbone is formed by the sugar-phosphate-sugar chain wherein the nitrogen bases are proposed almost perpendicular to this backbone, facing inwards. A and G of one strand base pairs with T and C respectively on the other strand. Between A and T there are two hydrogen bonds and between G and C, there are three hydrogen bonds wherein each strand appears as a helical staircase. One complete turn of the helical strand has ten base pairs
Q.3. Differentiate between nucleotide and nucleoside. Give two examples of each with their structure.
A.3. Following are the differences:
| Nucleoside | Nucleotide |
| It is a compound formed by the union of the nitrogenous base with a pentose sugar. They are slightly basic in nature. They are the components of the nucleotide and forms with deoxyribose and ribose Example – Uridine, Adenosine, Thymidine | It is a compound formed by the union of a nitrogen base, phosphate, and a pentose sugar. It is acidic in nature and is formed through phosphorylation of nucleoside. Example – GMP, CMP, AMP, UMP |
Q.4. Explain the different forms of lipids with some examples.
A.4. Lipids are not soluble by water and could exist as simple fatty acids. A fatty acid has a carboxyl group linked to an R-group, which could be methyl or ethyl or higher number of -CH2 groups. For instance, Arachidonic acid has 20 carbon atoms inclusive of the carboxyl carbon, the palmitic acid has 16 carbons inclusive of the carboxyl carbon. These fatty acids could be unsaturated or saturated. Another lipid is trihydroxy propane or glycerol.
Several lipids have fatty acids and glycerol wherein the fatty acids are esterified with glycerol, which results in diglycerides, triglycerides, monoglycerides and are known as oils and fats depending upon the melting point. Few other lipids have phosphorylated organic compound and phosphorous contained in them, which are phospholipids that are found in the cell membrane. Example – Lecithin.
Q.5.What are the main functions of carbohydrates?
A.5.The main function of carbohydrates are:
- Regulation of blood glucose.
- Involved in fat metabolism and prevents ketosis.
- Provide energy and food to the body and to the nervous system.
- Functions as the basic component of food including fibre, starch, and sugars.
- They are the primary source of energy. Therefore involved in the breakdown of starch into glucose, and proteins to produce energy for metabolism.
